Patients with severe mitral regurgitation (MR) have limited treatment options and are often refused surgery due to the condition of their health or advanced age. Tendyne™ Transcatheter Mitral Valve Replacement (TMVR) is a revolutionary mitral valve replacement technology that offers a safe and effective treatment option when others are not available.
Alternative treatment options are needed
MR is the most common heart valve disease in developed countries. The condition can be debilitating, greatly reducing a patient’s ability to take part in activities they enjoy and may result in progression to heart failure.
Mitral valve surgery for symptomatic patients is strongly recommended in current national guidelines, yet data suggests that only 50% of patients with severe MR undergo conventional surgery due to prohibitively high risk.
Reasons why patients are denied surgery includes factors such as advancing age, multiple comorbidities, hostile chest, and high-risk surgical technique. Medical therapies can be used in patients who are deemed unsuitable for conventional mitral valve surgery, and these can help to relieve symptoms, but they do not rectify the underlying structural problem of a leaky mitral valve in the way surgery can.
An alternative to conventional mitral valve surgery is therefore needed to provide a much-needed minimally invasive treatment option in patients with severe MR.
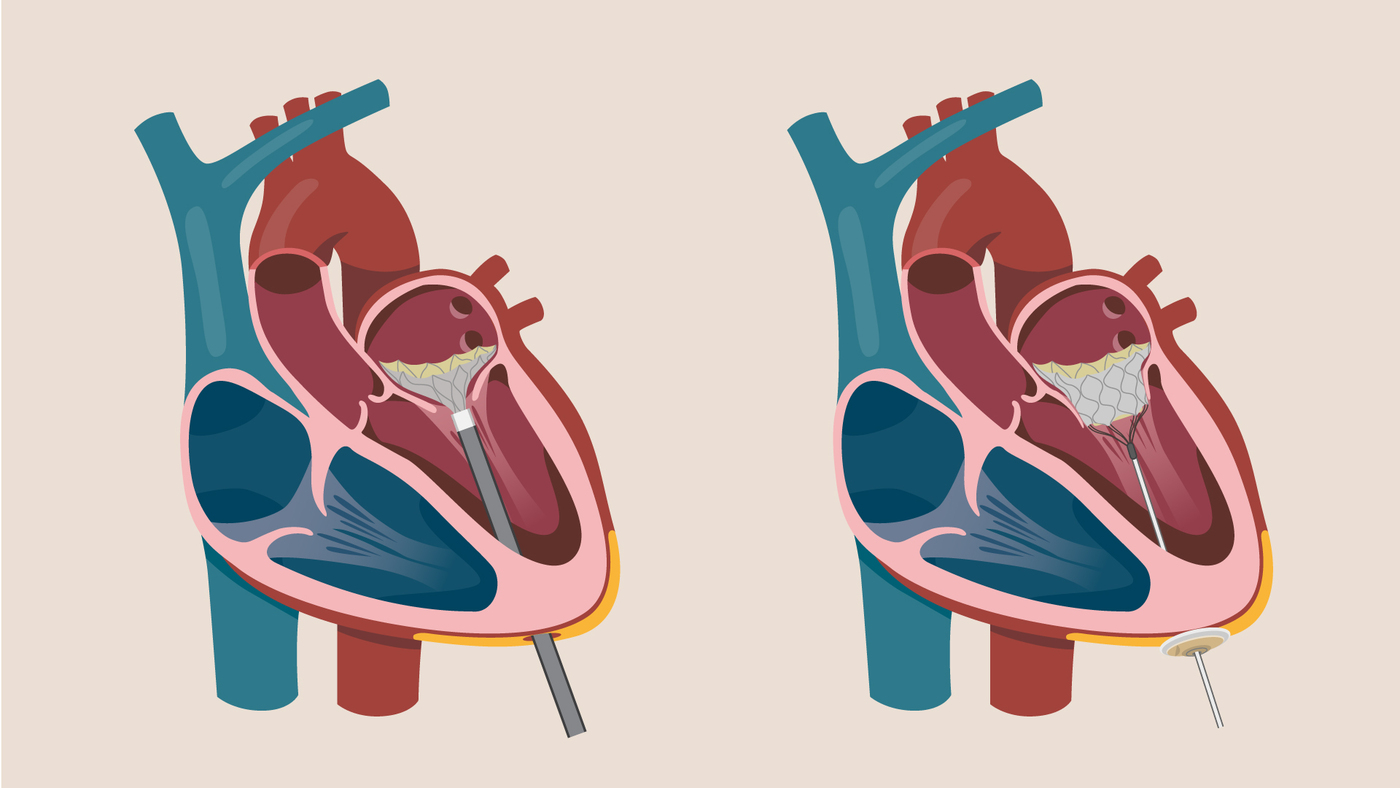
The Tendyne* TMVR valve is inserted into a patient’s beating heart with a catheter and secured in place with a tether that is attached to a pad (2) outside the heart
Tendyne: a first-in-class technology
Tendyne™ Transcatheter Mitral Valve Replacement (TMVR) is a ground-breaking technology that offers select patients with severe MR (≥ grade 3) the opportunity for a safe and effective replacement of their mitral valves.
The procedure is less invasive than conventional mitral valve surgery, requiring only a small 5cm incision between the ribs. Moreover, unlike conventional mitral valve surgery (which requires a patient’s heart to be stopped and the circulation supported on cardiopulmonary bypass), the Tendyne TMVR procedure facilitates insertion of the valve directly via a catheter into a patient’s beating heart with no requirement for cardiopulmonary bypass or sternotomy.
In addition, the procedure had a high 96% technical success rate reported and a significant improvement in symptom and quality of life measures. 82% of patients were in NHYA Class I/II at two years compared with 34% at baseline and there was an average 19-point increase in quality of life (KCCQ) scores over the same period.
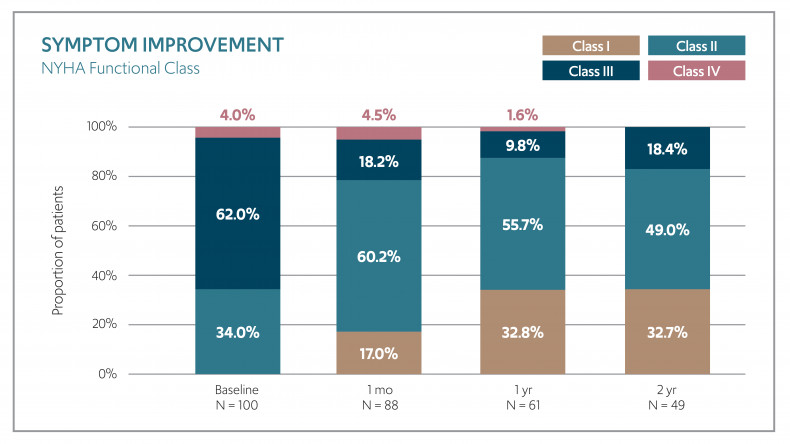
Symptom improvement - NYHA Functional Class
The future of mitral valve surgery
“This is a game-changer in mitral valve surgery. Patients who previously had limited treatment options now have one that is safe and effective in eliminating their mitral valve regurgitation,”
explains Dr Alison Duncan, Associate Specialist in Cardiology and Transcatheter Valve Therapies.
Dr Duncan is the UK Chief Investigator of the Tendyne Global Early Feasibility Study and performed the first procedure in the world with her surgical colleagues at The Royal Brompton Hospital in 2014. “I have patients who were previously suffering from heart failure due to mitral regurgitation who are now able to go the gym three times a week and are very active and full of life,” she says of her experiences.
Mr Cesare Quarto, consultant cardiac surgeon at The Royal Brompton and Harefield Hospitals was instrumental in pioneering the first Tendyne TMVR procedures and continues to do so. He says:
“We have the longest follow-up of patients of any centre in the world and we continue to see great safety and effectiveness. We are hopeful that its availability will be expanded so that it may benefit many more patients.”
As Royal Brompton Hospital is the only centre in the UK that offers the procedure, both Mr Quarto and Dr Duncan offer training for other clinicians and healthcare teams around the world in its use.
The technology gained its CE Mark in January 2020 and is the first of its kind available for wider use outside of clinical trials. It is awaiting NICE approval but is available privately at Guy’s and St Thomas’ Specialist Care.
*Tendyne is a trademark of Abbott or its related companies. Reproduced with permission of Abbott, © 2021. All rights reserved.